We wonder why there are billing concerns and prior authorization requirements with insurance companies for genetic or genomic testing that patients truly deserve and, when appropriate, changes outcomes substantially in patient care? Why would these organizations put...
Raising the Bar on Biomarker Test Accuracy Should Be Part of Access Efforts
Recently, under the banner of “access”, a trade association that represents a number of biomarker testing laboratories sent a letter to two Medicare administrators expressing concerns about the administrators’ proposals for tightening coverage on precision oncology...
Lab Owner Sentenced for $463 Million Genetic Testing Scheme
“In one of the largest genetic testing fraud cases ever tried to verdict, today’s sentence makes clear that the Department will seek justice for those who put profits above patient care, including owners and executives,” said Acting Assistant Attorney General Nicole...
What is Scientific Fraud and How can YOU report it to HHS Office of Inspector General?
𝗙𝗥𝗔𝗨𝗗 𝗜𝗡 𝗖𝗟𝗜𝗡𝗜𝗖𝗔𝗟𝗚𝗘𝗡𝗘𝗧𝗜𝗖𝗦 & 𝗚𝗘𝗡𝗢𝗠𝗜𝗖𝗦 𝗹𝗮𝗯𝘀: 𝟭) 𝗦𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝗳𝗿𝗮𝘂𝗱, 𝟮) 𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝘀𝗮𝗺𝗽𝗹𝗲 𝗿𝗲𝗰𝗿𝘂𝗶𝘁𝗶𝗻𝗴 𝗳𝗿𝗮𝘂𝗱, & 𝟯) 𝗯𝗶𝗹𝗹𝗶𝗻𝗴 𝗳𝗿𝗮𝘂𝗱. Do you work for a Clinical Genetics or Genomics laboratory that has a test that is reimbursed by the Centers for Medicare & Medicaid...
Genomic Data Heterogeneity across Molecular Diagnostic Laboratory – Published in The Journal of Molecular Diagnostics
“𝗘𝘃𝗲𝗻 𝗹𝗮𝗯𝗼𝗿𝗮𝘁𝗼𝗿𝗶𝗲𝘀 𝘄𝗶𝘁𝗵 𝗖𝗔𝗣 𝗮𝗰𝗰𝗿𝗲𝗱𝗶𝘁𝗮𝘁𝗶𝗼𝗻 𝗱𝗶𝗱 𝗻𝗼𝘁 𝗮𝗹𝘄𝗮𝘆𝘀 𝗳𝗼𝗹𝗹𝗼𝘄 𝗖𝗔𝗣 𝗿𝗲𝗾𝘂𝗶𝗿𝗲𝗺𝗲𝗻𝘁𝘀.” “𝗧𝗵𝗶𝘀 𝗽𝗶𝗹𝗼𝘁 𝘀𝘁𝘂𝗱𝘆 𝗱𝗲𝗺𝗼𝗻𝘀𝘁𝗿𝗮𝘁𝗲𝗱 𝘁𝗵𝗮𝘁 𝗹𝗶𝗺𝗶𝘁𝗮𝘁𝗶𝗼𝗻𝘀 𝗶𝗻 𝘁𝗵𝗲 𝗮𝗰𝗰𝘂𝗿𝗮𝗰𝘆, 𝘁𝗿𝗮𝗻𝘀𝗽𝗮𝗿𝗲𝗻𝗰𝘆, 𝗮𝗻𝗱 𝗰𝗼𝗻𝘀𝗶𝘀𝘁𝗲𝗻𝗰𝘆 𝗼𝗳 𝗴𝗲𝗻𝗲𝘁𝗶𝗰 𝗮𝗻𝗱 𝗴𝗲𝗻𝗼𝗺𝗶𝗰 𝗹𝗮𝗯𝗼𝗿𝗮𝘁𝗼𝗿𝘆 𝗿𝗲𝗽𝗼𝗿𝘁𝘀 𝗺𝗮𝗸𝗲 𝘁𝗵𝗲 𝘀𝘁𝗮𝗻𝗱𝗮𝗿𝗱𝗶𝘇𝗮𝘁𝗶𝗼𝗻, 𝗰𝗼𝗹𝗹𝗮𝘁𝗶𝗼𝗻, 𝗮𝗻𝗮𝗹𝘆𝘀𝗶𝘀,...
CLIAC – Clinical Laboratory Improvement Advisory Committee Next Generation Sequencing (NGS) Workgroup
Everyone from the genetic, genomic, lab, and pharmaindustry should at this point understand that there are “gaps” currently when assuring quality of NGS (Next Generation Sequencing) based testing in CLINICAL laboratory settings. This working group CLIAC (Clinical...
Natera – CareDx False Advertising Verdict Issued
Can labs that aren’t regulated by the FDA create whatever “claims” they wish pertaining to the performance of the test? YES! Does this false advertising ultimately hurt patients? YES! A recent article published by GenomeWeb stated that Natera and CareDx, Inc. are...
Experts Call for Better FDA Policing of Direct-to-Consumer Polygenic Risk Scores
Many DTC (Direct-to-Consumer) tests use a polygenic risk score (PRS) to ESTIMATE that individuals potential disease risk. The LACK of regulation in the Laboratory Developed Testing (LDT) also extends into a market that targets consumers for “wellness” testing. These...
The FDA has become “Increasingly Concerned” of LDTs
“The [FDA] has become increasingly concerned that some tests made by laboratories and not authorized by the FDA may not provide accurate and reliable test results or perform as well as FDA authorized tests. This may negatively impact treatment decisions.” – June 20th,...
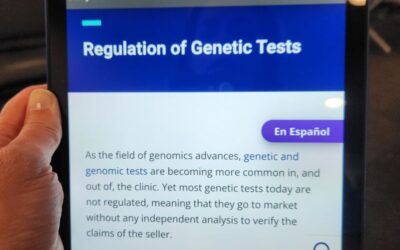
National Institue of Health Statment Confirms Lack of Regulation of Genetic Tests
“Most genetic tests today are not regulated, meaning that they go to market without any independent analysis to verify the claims of the seller.” – The National Institutes of Health https://lnkd.in/gcRf3Hg6. Most Precision Medicine is guided by genetic or genomic...